- General Information
- Health
- Society
- Media
- Become a MemberPREMIUM
- Events
- Contact Us
- Shop
- Categories
- Access to Health
- Ageing & Longevity
- Asset-Based Community Development
- Community & Health
- Ecology & Environment
- Economic & Political Root Causes
- Economic Stability
- Education
- Environmental Contaminants
- Ethnicity & Gender
- Health Condition of the Body
- Holistic Approach
- Law & Human Rights
- Men’s Health
- Natural Medicine
- Nutrition & Food
- On A Philosophical & Ethical Note
- Political Acceptance & Opposition
- Race
- Social Determinants of Health
- Traditional Medicine & Adjuvant Therapies
- Transhumanism
- Wellness
- Women’s Health
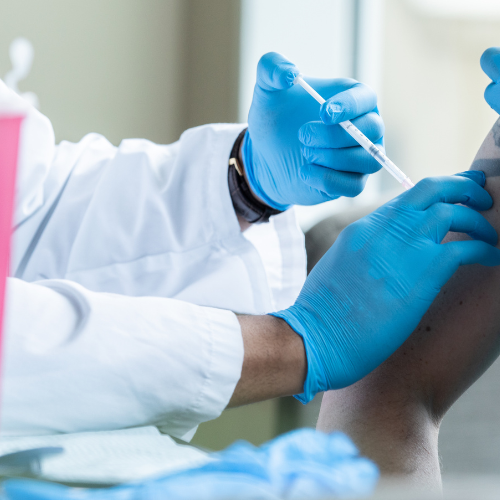
DOES INFLUENZA VACCINATION OF HEALTHCARE WORKERS PREVENT TRANSMISSION WITHIN CARE HOMES?
LESSONS LEARNED FOR VACCINATION AGAINST COVID-19 IN HCWS?
This review was co-authored by Dr. Frederik Feys.
INTRODUCTION
One of the most common types in human infection are the highly transmissible influenza respiratory viruses (types A, B and C) inducing mild to severe symptoms: fever, muscle pains, headaches, sore throat, respiratory aches and general fatigue. Influenza is a major cause of winter deaths in Europe and associated with high rates of mortality amongst OAs in LTRCs, known to be well-confined settings inducing rapid spread of the virus (Janafi et al. 2017). OAs are vulnerable due to comorbidities as existing chronic diseases become worse in intensity (Constantino and Vitale 2016).
VACCINES
Hay et al. (2001) argue that a major feature of influenza viruses is their ability to change genetic strains and develop into a novel virus subtype bypassing human immunity. As vaccinations are developed yearly, on basis of changing strains within a period of 6 months prior to the release of the vaccine, they might be ineffective. If strains in the vaccine are not matched sufficiently with the mutated virus, efficiency may be limited (Jiménez-Jorge et al. 2012).
OLDER ADULTS
The population under investigation are OAs being 60 years of age or older and residing in a long-term care institution. 65 years and older is the age-frame widely accepted to define people at greater risk of developing diseases and comorbidities (CDC 2017). Thomas, Jefferson and Lasserson (2016) suggest using 60 years of age as a benchmark as people also tend to seek placement in specialised facilities if prematurely struck by life-changing processes. Preference is given in this report using ‘older adults’ instead of ‘elderly people’ or ‘geriatric residents’.
VACCINE EFFICACY IN OLDER ADULTS
Influenza Outbreak Research
Camelloni et al. (2010) performed a cohort-study evaluating vaccine efficacy (VE) of a trivalent vaccination in one LTRC in Umbria (Italy). Despite vaccination, 43.4% became infected. The vaccine did not lead to the minimum of 60% level of protection as required. Potential flaws are noticed in the analyses. Recalibrating the non-parametric data to fit the t-test may have been unnecessary as a Wilcoxon/Man-U-Whitney-test would have fitted the purpose as it does not require the assumption of a normal distribution. Also, the use of a Chi-square is questionable as it follows the central limit theorem (Statsoft 2017).
During the 2011-2012 influenza season, three LTRCs in Spain were investigated by Castilla et al. (2012) after a reported influenza-like illness (ILI) outbreak. ILI is defined as the onset of a general symptom (fever and muscle aches) in combination with any respiratory symptom (cough, soreness of throat or respiratory problems). The vaccination rate ranged from 82% to 97% in 611 residents, the VE was estimated at 50% and 57 OAs were confirmed by laboratory testing to have total vaccine failure.
The retrospective cohort-study by Castilla et al. (2012) used non-parametric testing with the two-tailed Fisher’s exact test which is more defendable as an approach (du Prel et al. 2010) than the recalibration of data by Camilloni et al. (2010).
Immunosenescence or Failing to Convert to Antibodies
A decline in cellular response capacity leads to less immune responsiveness from the humoral system and is called immunosenescence (IS) (Haq and McElhaney 2014). Haralambieva et al. (2015) performed fundamental immunological laboratory research; investigating associations between vaccination, age and IS in 106 healthy OAs (50-74 years old) using blood samples and analysing them at baseline and after 3, 28 onto 75-days post-intervention. The seroconversion rate (immunity) was limited to 39% after 28 days. Researchers used non-parametric statistical analysis due to skewness in distributions. Wilcoxon sign rank test and Friedman rank sum were applied. To assess correlation, Spearman’s test was used with a p-value <0.05.
Covariates were analysed with ANCOVA. The median age of the sample was 59.7 years, with 61,3% males and 38.7% females. The strength of this study lies in the extensive investigation of humoral variables. This robust study underpins immunosenescence within a relatively young sample of OAs.
The more age increases, the more difficult seroconversion leads to protection (Haralambieva et al. 2015). Yang et al. (2020) found OAs having faces previous natural infections remain at high protective levels, enhanced by vaccination.
VE in OAs appears more limited than in younger populations. Informing stakeholders about the VE and promoting PPMs as an alternative can be considered (De Serres et al. 2017).
FLAWED DATA AND METHODOLOGICAL BIAS
Influenza vaccination in HCWs is recommended by the WHO, Centre for Disease Control and Prevention (CDC) in the USA and by the European Centre for Disease Prevention and Control (ECDC) within the European Union (EU). The CDC recommends all HCWs to be vaccinated as the ECDC aims at reaching 75% coverage within the EU (CDC 2016; ECDC 2017; WHO 2017b).
The WHO refers in its Seasonal Influenza Report on HCWs and the impact on OAs (WHO 2012) to four clustered-Randomized Controlled Trials (cRCTs); Carman et al. (2000) (643 citations), Hayward et al. (2006) (286 citations), Lemaitre et al. (2009) (129 citations) and Potter et al. (1997) (532 citations).
RCTs are considered the ‘golden standard’ in evidence hierarchy (Bothwell 2016), although cRCTs have reduced statistical efficiency (Donner and Klar 2004). Aforementioned cRCTs have been referenced a total of 1590 times in other scientific publications, hence the recommendations of the CDC and ECDC based on these WHO guidelines in vaccinating HCWs in LTRCs (CDC 2016; ECDC 2017).
Potter et al. (1997) recommend vaccinating HCWs in LTRCs as a preventative measure against influenza in OAs. A decrease in mortality was noted in the Carman et al. (2000) study which was published in The Lancet, where they evaluated the VE of HCWs and related mortality in OAs in LTRCs. Hayward et al. (2006) concluded vaccination of 8 HCWs could prevent 1 death among OAs residing in a LTRC. Finally, the fourth cRCT by Lemaitre et al. (2009) argued a 20% lower mortality of OAs within the vaccinated HCW group, supporting the call for overall vaccination of HCWs in LTRC.
Critique on the Four Most Cited Relevant cRCTs
It is observed that recent evidence undermines guidelines and needs addressing, it may change approaches in tackling viral outbreaks.
Evaluating the cRCTs using the ‘Grades of Recommendation’ by the Joanna Briggs Institute (2017) we conclude that the quality of the four aforementioned studies are weak as not all included cases of influenza were laboratory confirmed.
The Cochrane Database of Systematic Reviews published a review by Thomas, Jefferson and Lasserson (2016) entitled: “Influenza vaccination for healthcare workers who care for people aged 60 or older living in long-term care institutions”.
The authors identified a total of 2815 articles and after selection withheld five studies, amongst those the four cRTCs as previously cited. Selection bias was found in Potter et al. (1997) and Carman et al. (2000). Potter et al. (1997) included nearly 600 cases of suspected viral illness and suspected deaths related to influenza and deaths from all causes.
In both studies performance bias was detected as vaccination levels of HCWs only ranged from 33% to 67% of staff, also Lemaitre et al. (2009) may have been biased due to a vaccination level of 51% in HCWs. The Potter et al. (1997) study corrected statistically for clustering; the other three studies did not, which makes them vulnerable to statistical bias. Furthermore, no study applied a double-blinded approach. Thomas, Jefferson and Lasserson (2016) conclude a high risk of bias in all four studies as analysis of pooled data showed no evidence of positive outcome.
A review by De Serres et al. (2017) critically analysed the evidence presented by Thomas, Jefferson and Lasserson (2016) using this as a basis for further investigation. For their analysis, the authors looked at VE, outcomes and the mathematical principle of dilution of the four cRTCs.
De Serres et al. (2017) suggest that only laboratory-confirmed influenza infections should have been used as merely fever, coughs and other respiratory problems may occur through infection of other microorganisms. Authors further argue comorbidities contribute to ILI-related deaths and these figures will influence the basic mathematical principle of dilution.
They claim this principle to govern the inverse relationship between influenza-related disease reduction and the percentage of contribution of comorbidities. The more comorbidities contribute to the outcome, the lower the percentage reduction of the targeted intervention must be. This requires the percentage reduction of VE to be lower when comorbidities are included in a study outcome than when only influenza infection is calculated. They argue when two groups are compared evaluating the positive effects of vaccination; the reduction in percentage will be proportional to the absolute difference (AD) in coverage when comparing the two groups. Following formula is proposed (De Serres et al. 2017):
% reduction in outcome= (% VE) x (% influenza outcome) x (AD% in vaccine coverage)
The investigated cRCTs did not acknowledge this principle, which De Serres et al. (2017) consider a de facto major flaw and used this to recalculate the findings of the four cRCTs. They noticed percentage reductions for non-laboratory confirmed influenza were 8 times higher in the study of Hayward et al. (2006) compared to the predicted values (De Serres et al. fig.1). The other cRCTs also showed 3 to 4-fold higher results.
Fig 1.: Reported and predicted percentage reductions in patient outcomes between intervention and controls among cRTCs assessing indirect patient benefits from increased influenza vaccine coverage of healthcare workers in long- term care facilities. (Δ VC = AD in vaccine coverage; LCI = laboratory-confirmed influenza; ACM = all-cause mortality) (De Serres et al. 2017).
Evaluating indirect mortality prevention, possibly induced by the HCWs, they calculated the number of needed vaccinated individuals (NNV) using the figures of the previously mentioned cRCTs. The NNV is commonly defined as absolute risk reduction (Irwig, Irwig and Trevena 2008).
Number of unvaccinated HCWs
NNV= _____________________________________________________
Number of patient deaths preventable by HCWs vaccination
After their recalculation, De Serres et al. (2017) conclude HCWs vaccination could have only reduced the negative outcomes for 9%, leaving more than 80% of reductions unaccounted for. They further notice Hayward et al. (2006) to conclude that 8 HCWs should be vaccinated to prevent 1 death. De Serres et al. (2017) extrapolated this figure based on 5.5 million HCWs in the US, taking the principle of dilution and NNV into account and concluded almost 700.000 deaths could be prevented each year. This is unrealistic, knowing the specific mortality rates have never been higher than 49.000 for influenza in the US.
The authors were surprised that other scientific literature never used the NNV in reviewing existing data. De Serres et al. (2017) refute enforced vaccination of HCWs as evidence is insufficient to conclude this being of preventative importance for OAs in LTRCs.
As De Serres et al. (2017) state, the current recommendations are based on little evidence, therefore it is suggested to shift focus towards PPMs and keeping vaccination voluntary.
COST-EFFECTIVENESS OF INFLUENZA VACCINATION
A systematic review (Shields, Elvidge and Davies 2017) on health economics of seasonal vaccination in OAs included 8 articles out of 326 that were eligible to set criteria. Cost-effectiveness remains unconfirmed as they found methodological flaws and lack of robust data. Calculations ranged from €1,065 per quality-adjusted life-year (QALY) up to €572,305 per QALY.
Comparisons were difficult as studies used many different variables. Authors did not encounter one study that used the proposed WHO framework on health economics. Peasah et al. (2013) also conducted a systematic review; out of 27 studies only 8 reported a cost-effectiveness in vaccinating OAs based on the international accepted threshold of $50,000/QALY. Authors found no data from developing countries.
Cost-effectiveness is still very much a point of debate as researchers do not follow a standardized framework collecting data (Shields, Elvidge and Davies 2017). Hodgson et al. (2017) note the incremental cost-effectiveness ratio (ICER) should be less than £20,000 per QALY in the UK to be deemed cost- effective in influenza vaccination, whereas Brogan et al. (2016) argue $50,000 (£37,500) is acceptable in the US and Neumann, Cohen and Weinstein (2014) claim a threshold of $150,000 per QALY (£112,500) to be more realistic.
No studies have been retrieved evaluating cost-effectiveness in vaccinating HCWs to prevent infection in OAs.
SOCIAL FACTORS INFLUENCING VACCINE UPTAKE
Age, higher education levels, high income/social class, underlying diseases and known vaccinated peers are some of the predictors of higher uptake (Shaham et al. 2020). However, other research shows that higher status may not be the key driver as self-awareness of potential underlying risks is of great importance (Isler et al. 2020).
Within the population of HCWs believe of vaccine-efficacy, peer-pressure and facing chronic disease enhance vaccination rates. Personal reflections and perspectives take a role in developing underlying behavioural patterns. HCWs personally focusing on a healthy lifestyle see guidance in human physiology and the strength of natural ways of combatting flu-illness (Asma et al. 2016).
Furthermore, if the social context in which HWCs live shows a positive attitude towards vaccination HCWs data show a higher responsiveness. On the contrary leads critical scepticism to lower uptake (Konstantinou et al. 2021). Social networks, peer-information and peer-pressure appear to guidance decision making more than the science behind the vaccinations.
Although our analysis shows that influenza vaccination does not appear to be sufficient in preventing transmission to OAs, recent research about vaccine hesitancy is still based on the widely (falsely) accepted premises that influenza vaccination is a main driver in mitigating outbreaks. From this perspective behaviour is also evaluated in a recent review which concludes vaccine-hesitancy is driven by: false beliefs, disinformation, ‘the believe the natural immune system can handle it’, a.o. The supposition in the use of words by Guillari et al. (2021) as such seems biased as it starts form preconceived ideas around influenza-vaccination that have been refuted as our further analysis will show.
PSYCHOLOGICAL FACTORS AND RESPONSIVENESS TO INFLUENZA VACCINATIONS
A plethora of psychological and behavioural factors increase the risk of non- responsiveness to vaccines. For example, in OAs having an influenza vaccine, the immediate inflammatory response usually lasts a few days. But in those who are depressed it gets prolonged (Glaser et al. 2003). Studies (Glaser et al. 1998; Kiecolt-Glaser et al. 1996; Vedhara et al. 1999) found that stress reduced the secondary response to influenza vaccination. This is quite provocative.
It suggests that OAs confronted with an enduring and severe stressor develop antibodies to influenza immunizations at a slower rate (and possibly less antibody overall) than non-stressed control subjects. These data suggest that stress at immunization is associated with decreased protection up to 6 weeks after influenza immunization.
In a sample of OAs, bereavement in the year before getting a renewed influenza vaccination predicted a poorer antibody response after 1 month, whereas those who were married and had high marital satisfaction had a stronger antibody response (Phillips et al. 2006).
In previously vaccinated OAs, those with lower BMI had higher antibody responses, but only if they were also low in distress. Somehow surprisingly, distress was more strongly associated with larger post-vaccination interleukine-6 levels at older ages (~ 80 years) (Segerstrom et al. 2012).
The findings from Kohut et al. (2002) suggest that there is an association between physical activity, diet, psychosocial factors, and the immune response to influenza immunization in OAs; e.g. physically active OAs had better anti- influenza antibody levels. This benefit of being fit at older age was also found by Keylock et al. (2007).
In a study by Vedhara et al. (2003), influenza vaccination after 8 weeks of cognitive-behavioural stress management (sessions 1 hour a week) for chronically stressed OAs, a large effect was seen on antibody responses. Mindfulness-based stress reduction (MBSR) or exercise in a younger group of OAs (~60 years), yielded no significant antibody response improvements. However, both exercise and MBSR reduced the number of days sick from all- cause acute respiratory infection illness (Hayney et al., 2014). Yang et al. (2008) found 20 weeks of combined Taiji/Qigong meditation (3 x 1 hour sessions per week) in OAs resulted in improved antibody levels in comparison to a control waiting list group.
STRENGTHENING YOUR NATURAL IMMUNITY AGAINST INFLUENZA
The conventional Western immunity doctrine in which antigens lead to an immune response involves many molecules enhanced by plant and algal polysaccharides as described in Chinese medicine (Ai et al., 2020; Zhang et al. 2020).
Starch (e.g. potatoes, rice, peas, corn, beans) cellulose rich and chitin do not appear to have biological activity, rather high plant polysaccharides (also called non-starchy vegetables) like: spinach, artichoke, asparagus, broccoli, sprouts, cabbage, carrots, salad greens and tomato are proven to support your immune response (Zhang et al. 2020).
In a broader sense, Asian oriented herbal treatments seem to contribute to some extent in the prevention of influenza-like illnesses (Shahrajabian et al. 2020).
Western medicine primarily promotes physical exercise (Cao Dinh et al. 2017; Laddu et al. 2021) and some supplementary vitamins (Gombart et al. 2020; Pecora et al. 2020) although Wang et al. (2019) conclude in a review that evidence remains limited on the real-life impact of supplementary uptake of vitamins and micronutrients in strengthening the immune system.
Ayurveda medicine contributes on several levels within an integrative approach consisting of a decent sleep pattern combined with balanced food, physical exercise and attention for psychological wellbeing which combined lead to a better immune response (Thakur et al. 2021). Ayurveda as a holistic approach has some guiding evidence, however research also points towards limited knowledge of the physiological backgrounds (efficacy and safety) of some therapies (Umesh et al. 2021).
CONCLUSION
Influenza vaccination in HCWs shows little evidence-based underpinning as a preventative measure in controlling possible outbreaks or limiting mortality rates amongst OAs. Vaccine-uptake within HCWs should not be guided by social or peer-pressure.
Immunosenescence or the declining natural capacity to create antibodies, a process notably starting within age-specific group above 50, is hardly ever considered as a potential issue. Moreover, age appears strongly associated with declining counts of antibodies. We strongly suggest that healthcare providers recognize the effect of immunosenescence and the consequences this shall have on the efficiency of ILI-related vaccines within both the elderly and older HWCs.
Evidence-based practice should inform HCWs based on substantiated research. Unfortunately, many ILI-like vaccines campaigns and policies seem to be based on unsubstantiated science and require reevaluation. The latter should lead to the dissemination of correct EBM-evidence regarding vaccinating HCWs working in elderly care homes. We may assume these findings to be adequately valuable for the public at large, as some evaluated variables (workplace, age) are not necessarily workplace-specific.
We further suggest a focus on preventive approaches that aim at ameliorating natural immunity in populations without underlying diseases. Integrative health promotion should form the basis of any related policy.
Cost-benefit analysis on vaccinating HCWs as a preventative measure appear not to lead to many benefits for public health, nor for the individuals involved. There is an absolute need for further cost-benefit research aimed at evaluating preventative measures in reducing transmission of ILIs within vulnerable population groups.
BIBLIOGRAPHY
AI, H., WU, X., QI, M., ZHANG, L., HU, H., ZHAO, Q., ZHAO, J. & LIU, H. 2018. Study on the Mechanisms of Active Compounds in Traditional Chinese Medicine for the Treatment of Influenza Virus by Virtual Screening. Interdisciplinary Sciences: Computational Life Sciences, 10, 320-328.
ASMA, S., et al. 2016. Factors effecting influenza vaccination uptake among health care workers: a multi-center cross-sectional study. BMC Infectious Diseases, 16, 192.BARR, C. et al., 2011. A Process Evaluation of an Active Surveillance System for Hospitalized 2009-2010 H1N1 Influenza Cases: Journal of Public Health Management and Practice, 17(1), pp. 4-11
BARTHOLOMEW, L.K., PARCEL, G.S. and KOK, G., 1998. Intervention Mapping: A Process for Developing Theory and Evidence-Based Health Education Programs. Health Education & Behavior: The Official Publication of the Society for Public Health Education, 25(5), pp. 545-63
BEARD, J., OFFICER, A. and CASSELS, A., 2015. World Report on Ageing and Health. WHO Library Cataloguing-in-Publication Data. [online] Available from: http://apps.who.int/iris/bitstream/10665/186463/1/9789240694811_eng.pdf ?ua=1 [Accessed 25/10/2017]
BOSOMPRA, K., ASHIKAGA, T. and RUBY, A., 2004. Attitudes, Perceived Norms, and Intentions: A Needs Assessment Study of the Influenza Immunization Intentions of Elderly Citizens in Vermont. Journal of Rural Health, 20(2), pp. 125-30
BOTHWELL, L.E. et al., 2016. Assessing the Gold Standard – Lessons from the History of RCTs. The New England journal of medicine, 374(22), pp. 2175- 2181
BROKKEN, S., 2017. Timeframe for Intervention Planning and Process Evaluation. Unpublished. RGU, Aberdeen, UK.
CAMILLONI, B., NERI, M., LEPRI, E., BASILEO, M., SIGISMONDI, N., PUZELLI, S., DONATELLI, I. & IORIO, A. M. 2010. An influenza B outbreak during the 2007/2008 winter among appropriately immunized elderly people living in a nursing home. Vaccine, 28, 7536-41.
CARMAN, W. et al., 2000. Effects of influenza vaccination of health-care workers on mortality of elderly people in long-term care: a randomised controlled trial. The Lancet, 355(9198), pp. 93-7
CASTILLA, J. et al., 2012. Influenza outbreaks in nursing homes with high vaccination coverage in Navarre, Spain, 2011/12. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin, 17(14), pp. 20141
CAVANAGH, S. and CHADWICK, K., 2005. Health Needs Assessment: A Practical Guide. London – United Kingdom: National Institute for Health and Clinical Excellence.
CDC, 2016. Influenza vaccination information for health care workers. [online] Available from: https://www.cdc.gov/flu/pdf/toolkit/flu-vaccination-health- care-workers.pdf [Accessed 10/27 2017]
CDC, 2017. People at High Risk of Developing Flu–Related Complications. [online] Available from: https://www.cdc.gov/flu/about/disease/high_risk.htm [Accessed 27/10/ 2017]
CONSTANTINO, C. and VITALE, F., 2016. Influenza vaccination in high-risk groups: a revision of existing guidelines and rationale for an evidence-based preventive strategy. Journal of Preventive Medicine and Hygiene, 57(1), pp. E13-E18
DATTA, J. and PETTICREW, M., 2013. Challenges to evaluating complex interventions: a content analysis of published papers. BMC Public Health, 13, pp. 568-568
DE SERRES, G. et al., 2017. Influenza Vaccination of Healthcare Workers: Critical Analysis of the Evidence for Patient Benefit Underpinning Policies of Enforcement. PloS one, 12(1), pp. e0163586
DONNER, A and KLAR, N., 2004. Pitfalls of and controversies in cluster randomization trials. American Journal of Public Health: JPH, 94(3), pp. 416- 22
DU PREL, J. et al., 2010. Choosing Statistical Tests. Deutsches Ärzteblatt International, 107(19), pp. 343–8
ECDC, 2017. Seasonal influenza vaccination in Europe: Vaccination recommendations and coverage rates in the EU Member States for eight influenza seasons (2007-2008 to 2014-2015). Stockholm: European Centre for Disease Prevention and Control.
ECHEVERRÍA, M., JIMENEZ-MOLINA, A. and RÍOS, S.A., 2015. A Semantic Framework for Continuous U-health Services Provisioning. Procedia Computer Science, 2015, 60, Supplement C, pp. 603-612
EMA, 1997. Note for guidance on harmonization of requirements for influenza vaccines. 1997. [online] European Agency for the Evaluation of Medicinal Products, London, United Kingdom. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin e/2009/09/WC500003945.pdf [Accessed 25/10/2017]
GOMBART, A. F., PIERRE, A. & MAGGINI, S. 2020. A Review of Micronutrients and the Immune System–Working in Harmony to Reduce the Risk of Infection. Nutrients, 12, 236.
GLASER, R., KIECOLT-GLASER, J. K., MALARKEY, W. B. & SHERIDAN, J. F. 1998. The influence of psychological stress on the immune response to vaccines. Ann N Y Acad Sci, 840, 649-55.
GREEN, J. and TONES, K., 2010. Health Promotion: Planning and Strategies. London – p. 162-167: SAGE Publications.
HAND, M.W. and SEIBERT, S.A., 2016. Linking Root Cause Analysis to Practice Using Problem-Based Learning: Nurse educator, 41(5), pp. 225-7
HAYNEY, M. S., COE, C. L., MULLER, D., OBASI, C. N., BACKONJA, U., EWERS, T. & BARRETT, B. 2014. Age and psychological influences on immune responses to trivalent inactivated influenza vaccine in the meditation or exercise for preventing acute respiratory infection (MEPARI) trial. Hum Vaccin Immunother, 10, 83-91.
HAQ, K. and MCELHANEY, J.E., 2014. Immunosenescence: influenza vaccination and the elderly. Current opinion in immunology, 29(1), pp. 38-42
HARALAMBIEVA, I.H. et al., 2015. The impact of immunosenescence on humoral immune response variation after influenza A/H1N1 vaccination in older subjects. PloS one, 10(3), pp. e0122282
HAY, A. et al., 2001. The evolution of human influenza viruses. Philosophical Transactions of the Royal Society B: Biological Sciences, 356(1416), pp. 1861-70
HAYWARD, A.C. et al., 2006. Effectiveness of an influenza vaccine programme for care home staff to prevent death, morbidity, and health service use
among residents: cluster randomised controlled trial. BMJ: British Medical Journal / British Medical Association, 333(7581), pp. 1241
HODGSON, D. et al., 2017. Effect of mass paediatric influenza vaccination on existing influenza vaccination programmes in England and Wales: a modelling and cost-effectiveness analysis.
IRWIG, L., IRWIG, J. and TREVENA, L., 2008. Relative risk, relative and absolute risk reduction, number needed to treat and confidence intervals. Smart Health Choices: Making Sense of Health Advice. London: Hammersmith Press. pp. 228-237
ISLER et al., 2020. Limits of the social-benefit motive among high-risk patients: a field experiment on influenza vaccination behaviour. BMC Public Health, 20(1), 240.
JACOBS, J. et al., 2012. Tools for implementing an evidence-based approach in public health practice. Preventing chronic disease, 9, pp. E116
JBI, 2014. The JBI approach [online] Adelaide – Australia: Joanna Briggs Institute – The University of Adelaide. Available from: http://joannabriggs.org/jbi- approach.html#tabbed-nav=Grades-of-Recommendation [Accessed 30/11/ 2017]
JIMENEZ-JORGE, S. et al., 2012. Early estimates of the effectiveness of the 2011/12 influenza vaccine in the population targeted for vaccination in Spain, 25 December 2011 to 19 February 2012. EuroSurveillance, 17(12).
KEYLOCK, K. T., LOWDER, T., LEIFHEIT, K. A., COOK, M., MARIANI, R. A., ROSS, K., KIM, K., CHAPMAN-NOVAKOFSKI, K., MCAULEY, E. & WOODS, J. A. 2007. Higher antibody, but not cell-mediated, responses to vaccination in high physically fit elderly. J Appl Physiol (1985), 102, 1090-8.
KIECOLT-GLASER, J. K., GLASER, R., GRAVENSTEIN, S., MALARKEY, W. B. & SHERIDAN, J. 1996. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci U S A, 93, 3043- 7.
KOHUT, M. L., COOPER, M. M., NICKOLAUS, M. S., RUSSELL, D. R. & CUNNICK, J. E. 2002. Exercise and psychosocial factors modulate immunity to influenza vaccine in elderly individuals. J Gerontol A Biol Sci Med Sci, 57, M557-62.
KOK, G. et al., 2011. Planning for influenza vaccination in health care workers: An Intervention Mapping approach.
KONSTANTINOU, P., GEORGIOU, K., KUMAR, N., KYPRIANIDOU, M., NICOLAIDES, C., KAREKLA, M. & KASSIANOS, A. P. 2021. Transmission of Vaccination Attitudes and Uptake Based on Social Contagion Theory: A Scoping Review. Vaccines, 9, 607.
LEMAITRE, M. et al., 2009. Effect of Influenza Vaccination of Nursing Home Staff on Mortality of Residents: A Cluster-Randomized Trial. Journal of the American Geriatrics Society, 57(9), pp. 1580-6
LI, S. and MACKANESS, W.A., 2015. A multi-agent-based, semantic-driven system for decision support in epidemic management. Health informatics journal, 21(3), pp. 195-208
MARSEILLE, E. et al., 2015. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bulletin of the World Health Organization, 93(2), pp. 118-24
MAYCOCK, B., HOWAT, P. and SLEVIN, T., 2001. A decision-making model for health promotion advocacy: the case for advocacy of drunk driving control measures. Promotion & education, 8(2), pp. 59-64
MOORE, G.F. et al., 2015. Process evaluation of complex interventions: Medical Research Council guidance. BMJ (Clinical research ed.), 350, pp. h1258- h1258
NAJAFI, M. et al., 2017. The Effect of Individual Movements and Interventions on the Spread of Influenza in Long-Term Care Facilities. Medical Decision Making, 37(8), pp. 871-881
NEUMANN, P.J., COHEN, J.T. and WEINSTEIN, M.C., 2014. Updating Cost- Effectiveness — The Curious Resilience of the $50,000-per-QALY Threshold. New England Journal of Medicine, 371(9), pp. 796-7
OFSTEAD, C.L. et al., 2017. Moving the needle on nursing staff influenza vaccination in long-term care: Results of an evidence-based intervention. Vaccine, 35(18), pp. 2390-2395
PEASAH, S.K. et al., 2013. Influenza cost and cost-effectiveness studies globally – A review. Vaccine, 31(46), pp. 5339-48
PHILLIPS, A. C., CARROLL, D., BURNS, V. E., RING, C., MACLEOD, J. & DRAYSON, M. 2006. Bereavement and marriage are associated with antibody response to influenza vaccination in the elderly. Brain Behav Immun, 20, 279-89.
POTTER, J. et al., 1997. Influenza Vaccination of Health Care Workers in Long- Term-Care Hospitals Reduces the Mortality of Elderly Patients. Journal of Infectious Diseases: Official Publication of the Infectious Diseases Society of America, 175(1), pp. 1-6
QUIGLEY, R. et al., 2004. Clarifying health impact assessment, integrated impact assessment and health needs assessment. [online] Australia: hiaconnect. Available from: http://hiaconnect.edu.au/old/files/Clarifying_Hia.pdf [Accessed 30/11/2017]
RAVIOTTA, J.M. et al., 2017. Cost-effectiveness and public health impact of alternative influenza vaccination strategies in high-risk adults.
RIPHAGEN-DALHUISEN, J. et al., 2013. Planning and process evaluation of a multi-faceted influenza vaccination implementation strategy for health care workers in acute health care settings. BMC Infectious Diseases, 13(1), pp. 235
ROLLER-WINSBERGER, R. et al, 2021. The role of health determinants in the influenza vaccination uptake among older adults (65+): a scope review. Aging Clinical and Experimental Research, 33(8), 2123-2132
SEGERSTROM, S. C., HARDY, J. K., EVANS, D. R. & GREENBERG, R. N. 2012. Vulnerability, distress, and immune response to vaccination in older adults. Brain Behav Immun, 26, 747-53.
SEMANTIC SOFTWARE, 2017. The holy grail of artificial intelligence. [online] Sydney, Australia: Available from: http://semanticsoftware.com.au/semantic-computing/ [Accessed 30/11/ 2017]
SHABAN-NEJAD, A. et al., 2017. A Semantic Framework for Logical Cross- Validation, Evaluation and Impact Analyses of Population Health Interventions. Studies in health technology and informatics, 235, pp. 481- 485
SHAHAM, A. et al., 2020. Personal and social patterns predict influenza vaccination decision. BMC Public Health, 20(1), 222.
SHAHRAJABIAN, M. H., SUN, W. & CHENG, Q. 2020. Traditional Herbal Medicine for the Prevention and Treatment of Cold and Flu in the Autumn of 2020, Overlapped With COVID-19. Natural Product Communications, 15, 1934578X20951431.
SHIELDS, G.E., ELVIDGE, J. and DAVIES, L.M., 2017. A systematic review of economic evaluations of seasonal influenza vaccination for the elderly population in the European Union. BMJ Open, 7(6), pp. e014847
STATSOFT, 2017. Elementary concepts in statistics. [online] Palo Alto – USA: TIBCO Software. Available from: http://www.statsoft.com/Textbook/Elementary-Statistics-Concepts [Accessed 25/10/2017]
STEVENS, A., GILLAM, S. and WRIGHT, J., 1998. Needs assessment: from theory to practice. BMJ: British Medical Journal / British Medical Association, 316(7142), pp. 1448-52
THAKUR, R., NAIK, R., MYTHREY, R. & BHAT, S. 2021. Ayurveda perspectives and research updates on factors influencing the immunity: A review. Journal of Indian System of Medicine, 9, 21.
THOMAS, R.E., JEFFERSON, T. and LASSERSON, T.J., 2016. Influenza vaccination for healthcare workers who care for people aged 60 or older living in long- term care institutions. The Cochrane database of systematic reviews, (6):CD005187. doi(6), pp. CD005187
UNITED NATIONS, 2015. World population ageing. [online] New York: United Nations – Department of Economic and Social Affairs Population Division. Available from: http://www.un.org/en/development/desa/population/publications/pdf/ageing /WPA2015_Report.pdf [Accessed 22/10/2017]
VEDHARA, K., BENNETT, P. D., CLARK, S., LIGHTMAN, S. L., SHAW, S., PERKS, P., HUNT, M. A., PHILIP, J. M. D., TALLON, D., MURPHY, P. J., JONES, R. W., WILCOCK, G. K. & SHANKS, N. M. 2003. Enhancement of Antibody Responses to Influenza Vaccination in the Elderly following a Cognitive- Behavioural Stress Management Intervention. Psychotherapy and Psychosomatics, 72, 245-252.
VEDHARA, K., COX, N. K., WILCOCK, G. K., PERKS, P., HUNT, M., ANDERSON, S., LIGHTMAN, S. L. & SHANKS, N. M. 1999. Chronic stress in elderly carers of dementia patients and antibody response to influenza vaccination. Lancet, 353, 627-31.
WANG, M. X., KOH, J. & PANG, J. 2019. Association between micronutrient deficiency and acute respiratory infections in healthy adults: a systematic review of observational studies. Nutrition Journal, 18, 80
WHO, 2012. Influenza position paper: Grading of scientific evidence (healt care workers – impact on elderly patients). [online] WHO. Available from: http://www.who.int/immunization/policy/position_papers/influenza/en/ [Accessed 27/10/2017 2017]
WHOa, 2016. Ageing and health – commit to action. [online] WHO. Available from: http://www.who.int/ageing/commit-action/en/ [Accessed 25/10/2017 2017]
WHOb, 2016. Fact sheet: influenza (seasonal).
[online] WHO. Available from: http://www.who.int/mediacentre/factsheets/fs211/en/ [Accessed 24/10/ 2017]
WHO, 2017. Global influenza surveillance and response system (GISRS). [online] WHO. Available from: http://www.who.int/influenza/gisrs_laboratory/en/ [Accessed 24/10/2017]
YANG, B., LESSLER, J., ZHU, H., JIANG, C. Q., READ, J. M., HAY, J. A., KWOK, K. O., SHEN, R., GUAN, Y., RILEY, S. & CUMMINGS, D. A. T. 2020. Life course exposures continually shape antibody profiles and risk of seroconversion to influenza. PLoS Pathog, 16, e1008635.
YANG, Y., VERKUYLEN, J., ROSENGREN, K.S., MARIANI, R.A., REED, M., GRUBISICH, S.A., WOORDS, J.A., SCHLAGHAL, B., 2008. Effects of a Traditional Taiji/Qigong Curriculum on Older Adults’Immune Response to Influenza Vaccine, in: HONG, Y. (Ed.), Medicine and Sport Science. KARGER, Basel, pp. 64–76.
Disclaimer
The views and analysis expressed in this content (video, blog, article, etc.) are solely that of the author and does not necessarily reflect the views of humani-well, the organisation, or other associated parties.
Medical disclaimer: you should not rely on the information on this website as a replacement for seeing a doctor or other qualified healthcare provider for a diagnosis or treatment. Nothing in this publication should be interpreted as a substitute for the advice of a qualified healthcare provider. Please consult a medical professional before using any information contained in this publication.
Drs. Sam Brokken
Next to guiding this bunch of great people at Humani-Well, Sam covers a broad range of topics. From biomedical issues to societal impact analysis. HIs biggest strength? Being a 'generalist, and master of none'. Know More
© 2025 Humaniwell | CHOOSE WISELY. Powered by NoCodeLabs.